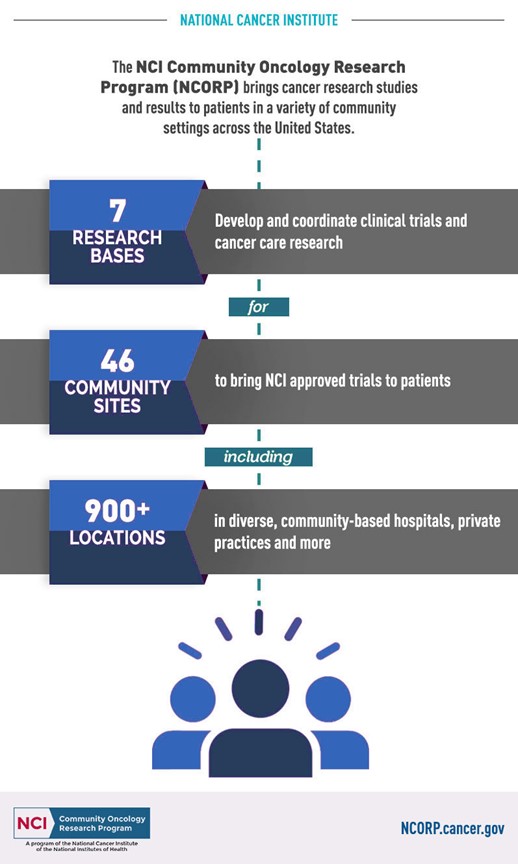
Supporting Cancer Research in Our Community
Nevada • Arizona • California • Washington
Nevada NCORP is a non-profit dedicated to promoting state-of-the-art cancer treatment for patients in our community. We bring clinical trials to participating clinics and hospitals to advance new treatment, screening, and prevention options.

Find a Clinical Trial
We support cancer research in four western states and new clinical trials are updated daily. Search the trials for openings based on cancer type, age, location and more.
Affiliated Hospitals and Clinics
We work with more than 50 hospitals and clinics in Nevada, Washington, Arizona and California to bring cancer research to our communities. Find local participants near you.

What We Do
The Nevada NCORP makes it possible for affiliated community oncology clinics to offer NCI funded cancer research studies to their patients. Our goal is to give patients access to investigational therapies without having to leave the community.

Coordinate local research studies
We support dedicated research coordinators in affiliated community hospitals and clinics and provide data management and regulatory requirements of conducting research minimizing the impact of research on busy clinic physicians and nurses.

Support Researchers
We arrange for patient data to connect with national research labs and biobanks and provide timely information to the national trial teams for analysis. Our affiliated physicians review and comply with all study requirements to ensure that patient data can be used to advance studies.

Educate Patients & Doctors
We provide research study participants and their physicians with updates as they are released, distribute informational materials to hospitals and clinics, and connect patients and physicians with available research for cancer, symptom management, cancer prevention and cancer screening.
Distribute Funding
We track funded clinical research study activities through the National Clinical Trials Network and distribute funding as directed by the study sponsor to select clinics and hospitals in Nevada, Arizona, California and Washington.
We need your help
For over 40 years, the Nevada NCORP has been committed to serving small communities in Nevada and beyond by bringing cutting-edge NCI cancer research directly to patients of all ages and their doctors, in rural areas that might otherwise lack access to these vital studies.
Your donation will help us continue to provide this important service to patients and doctors in our community.
Frequently Asked Questions
Your primary care doctor or insurance company will help you to choose a cancer doctor, also called an oncologist. The Nevada NCORP does not employ doctors, we work with oncologists who are at clinics and hospitals in the community.
The Nevada NCORP is accepting new physicians. If you doctor is interested in joining us there is information on our Affiliated Hospitals and Clinics page.
Clinical trials are research studies that involve people. Clinical trials are the way that doctors find ways to improve treatments and quality of life for people who have or may develop certain diseases.
Treatment trials test new drugs or new ways to use current drugs, vaccines, perform surgery, or deliver radiation therapy to improve the effectiveness of cancer treatment.
Symptom Management trials to find better ways to manage the effects of cancer or the side effects of cancer treatments.
Prevention trials test a new treatment or lifestyle change that may lower the risk of getting a specific cancer.
Screening trials test a new or different way of detecting cancer.
Tissue collection trials test new ways of identifying cancer characteristics to make treatments more effective or personalized.
The ethical and legal principles for medical practice also apply to clinical trials. Also, National Cancer Institute (NCI) approved clinical research is federally regulated with many required protections for participants.
Every clinical trial in the U.S. must be approved and monitored by an Institutional Review Board (IRB) to make sure the risks are as low as possible and are worth any potential benefits.
An IRB can stop a clinical trial if it appears to be causing unexpected harm to the patients.
For more information please visit:
https://www.cancer.gov/about-cancer/treatment/clinical-trials/patient-safety
Costs of care may be covered by private or public insurance companies or in some cases a clinical trial sponsor. Before joining a clinical trial it is important to discuss the costs with your healthcare provider. The consent form with provide information about costs before joining a clinical trial.
Every clinical trial has an action plan called a protocol, that describes what will be done in the trial and why each part of the trial is needed. Each protocol includes guidelines called eligibility criteria. The eligibility criteria explain who can and cannot participate.
Eligibility criteria has many factors which may include age, sex, medical history, and current health status. For cancer treatment trials eligibility may include the type and stage of cancer, and the type(s) of cancer treatment already received. Your doctor will make sure the study is right for you before you begin.
A placebo is an inactive pill, liquid, powder or injection that has no treatment value.
If a placebo is used by itself, it is because no standard treatment exists. In such a case, a trial would compare a new treatment to a placebo.
Mostly placebos are given along with a standard treatment. Example: a trial may compare a standard drug plus a new drug to the effects of the same standard drug plus a placebo.
When you consent join a study the treatments will be explained to you in detail, you should understand the treatment possibilities before you agree to join the study.